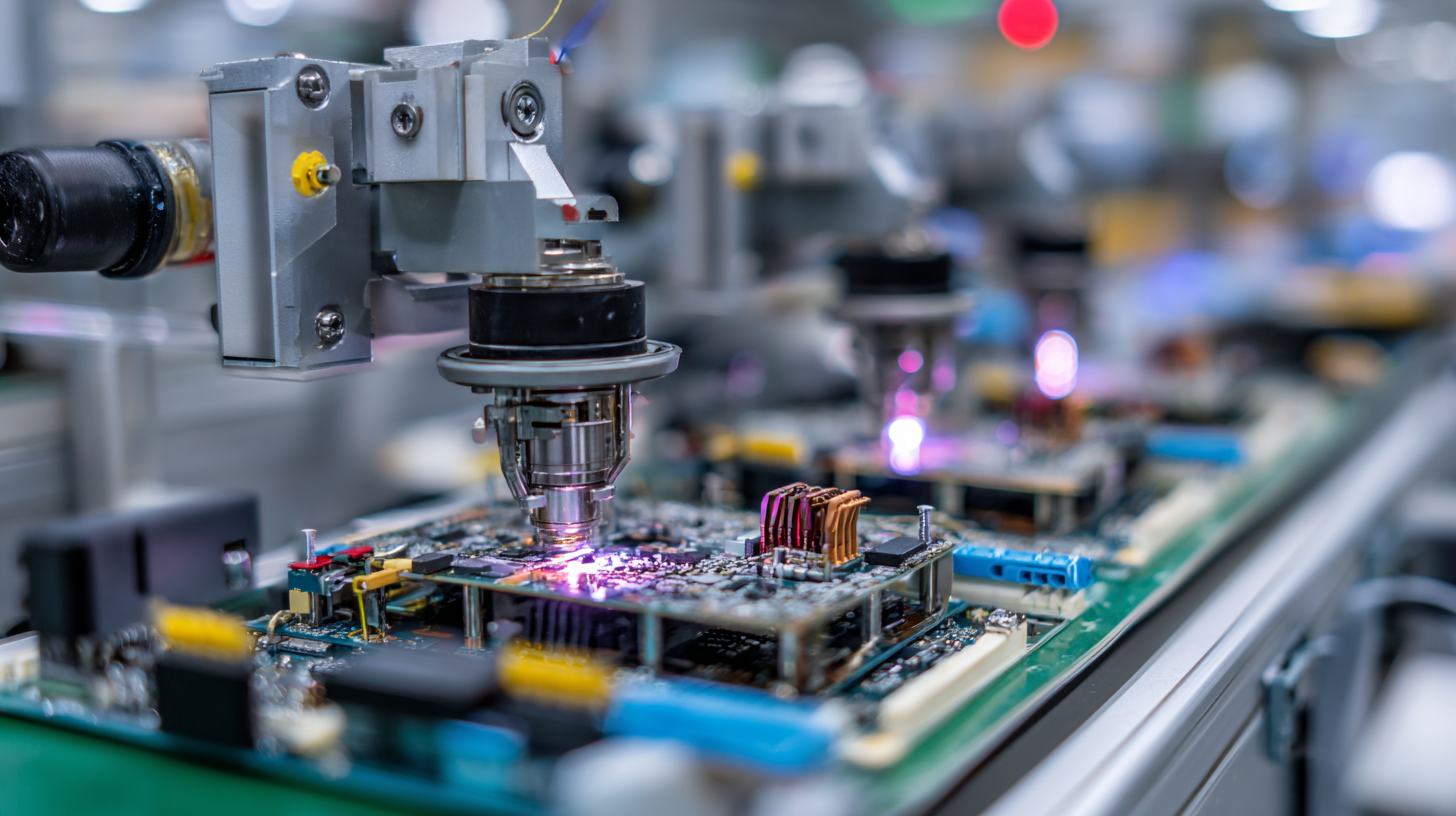
Most medical devices don’t die in the clinic; they die on the factory floor or in the audit room. MD&M West is where the survivors learn to scale—design controls that don’t suffocate speed, automation that passes validation, and supply chains that keep their nerve. This year, the Anaheim vibe: software eats the shop floor.
What the smartest teams shipped
- Closed‑loop assembly with inline vision that logs defects straight into the eQMS so CAPA isn’t a quarterly panic.
- Digital work instructions with step‑verification, making line changes auditable and training times less medieval.
- Human factors by design, not afterthought: summative testing embedded in agile sprints, with formative feedback flowing back into tooling changes within days—not quarters.
- SaMD and firmware change control wired into product lifecycle systems—model cards, target‑performance specs, and cybersecurity bills of materials shipped by default.
Supply chain realism
Sterilization capacity (EtO, e‑beam, gamma) remains the pacing item. Teams that pre‑qualified dual sterilization routes and validated accelerated aging are months ahead. On materials, the migration to recyclable mono‑materials is real—but dose accuracy and needle safety remain non‑negotiable.
The Anaheim checklist for founders
- Arrive with process capability indices, not just cost curves.
- Show supplier qualification depth and your plan for MDR/IVDR annexes if you touch Europe.
- Bring your post‑market surveillance loop: complaint coding, trend detection, and how you’ll trigger design changes without CHAOS.
Dates & place: 4–6 February 2025, Anaheim Convention Center.